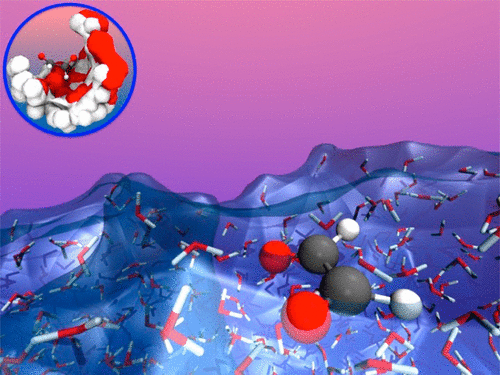
Interfacial chemistry involving glyoxal at aerosol surfaces is postulated to catalyze aerosol growth. This chemistry remains speculative due to a lack of detailed information concerning the physicochemical behavior of glyoxal at the interface of atmospheric aerosols. Here, we report results from high-level electronic structure calculations as well as both classical and Born–Oppenheimer ab initio molecular dynamics simulations of glyoxal solvation at the air/liquid water interface. When compared to the gas phase, the trans to cis isomerization of glyoxal at the liquid water interface is found to be catalyzed; additionally, the trans conformation is selectively solvated within the bulk to a greater degree than is the cis conformation. These two processes, i.e., the catalytic effect at the water interface and the differentially selective solvation, act to enhance the concentration of the cis isomer of glyoxal at the water interface. This has important consequences for the interpretation of experiments and for the modeling of glyoxal chemistry both at the interface of water clouds and at aerosols. Broader implications of this work relate to describing the role of interfaces in selecting specific stereo molecular structures at interfacial environments. The full article can be found at https://pubs.acs.org/doi/abs/10.1021/jacs.6b10208.